Shanghai, China, April 12th, 2023 – Shanghai Immunocan Biotech Co., Ltd._
In December 2022, Shanghai Immunocan Biotech Co., Ltd. launched ImmuMab® mouse, a fully human antibody discovery platform based on patented gene editing technology, and initiated mouse evaluation and application projects. Thus far, the four projects from different antigens have demonstrated stable immune responses as wild type mice, laying a solid foundation for further development of therapeutic antibodies.
First-generation transgenic mice were employed for therapeutic monoclonal antibody development in the 1990s, producing the first FDA-approved transgenic mouse-derived fully human monoclonal antibody drug, Panitumumab, in 2006. Through continual optimization and upgrading, transgenic mice across the globe have contributed dozens of FDA-approved antibody drugs, making up the largest part of fully human antibody technologies. The remarkable achievement has led to early-stage transgenic mice being acquired by pharmaceutical companies such as BMS and Amgen, transformed as internal discovery platforms there. On the other hand, some of the accessible transgenic mouse strains in the industry today are still facing challenges of unstable immune responses, low titers, or insufficient antibody diversity.
Immunocan has utilized its independently developed efficient Massive-fragment across species in situ replacement technology (MASIRT®) to construct ImmuMab® mouse, a gene-edited fully human antibody discovery mouse. The complete in situ replacement of variable regions of heavy or light chains can be achieved within single operation. The “single-step operation” brought intact human sequences, while the “in situ replacement” maintains integrity of the mouse genome. These technical advantages allow of human antibody genes in mouse keeping functional regulating environment required for transcription while reducing the impact of humanization on mouse physiological and developmental functions. As a result, advanced MASIRT® helps ImmuMab® Mouse preserve their natural optimizing process of stable antibodies, thus enabling the production of highly diverse and high-affinity fully human antibody repertoires.
So far, the four immunizing-completed antigens vary in structures, including single transmembrane, multiple transmembrane, human Fc fusion and mouse Fc fusion etc. Mice from the four projects generated stable immune responses, even surpassing some wild-type strains, such as in project of antigen A. Existing data demonstrate the high efficiency and stability of ImmuMab® mouse in fully human antibody discovery.
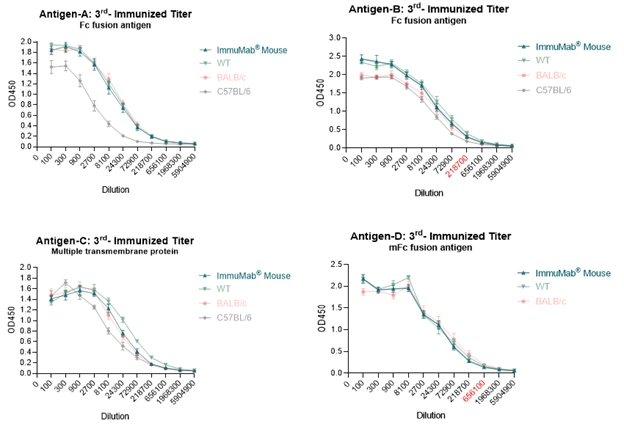
Immunocan will partner on ImmuMab® mice in development of several fully human antibodies, ADCs, and cell therapy drugs and at the same time optimize and expand the company’s multispecies antibody discovery capabilities through MASIRT® technology. Leveraging inherent functional advantages from in vivo process and low immunogenicity from fully human sequences, Immunocan is empowering the development of innovative biological drug discovery and combination therapies.
For more information, please contact
Massive-fragment Across Species In situ Replacement Technology (MASIRT®)
Immunocan’s original core technology: Mb-scale massive-fragment across species in situ replacement technology in a single operation. It allows for rapid construction of antibody discovery platforms from multiple species such as rabbit in addition to mouse.
About ImmuMab® Mouse platform
ImmuMab® Mouse is the first human antibody animal platform created by Immunocan based on its proprietary MASIRT® technology. We have completed the construction of a fully human mouse platform in 18 months based on our proprietary massive-fragment gene replacement technology, achieving Mb-scale large fragment gene replacements. Available data suggest that the platform can generate antibodies with lower immunogenicity without the need for lead antibody optimization or later humanization. The company has filed relevant patents for the platform.
About Immunocan
Founded in 2020, Shanghai Immunocan Biotech Co., Ltd. is a biotechnology company dedicated to building antibody drug discovery platforms using innovative gene editing technology, MASIRT®. Our goal is to empower global antibody drug R&D with proprietary gene-edited animal platform generating human and alpaca antibody, suitable for different drug discovery scenarios.

